Research
Transcriptional regulatory mechanisms involve a complex interplay of factors whose effects are mediated by physical interactions among them and diverse enzymatic activtites, all occurring in the context of the native chromatin fiber. To understand aspects of this regulation we apply approaches that span the full range from biochemistry, biophysics and structural biology, to molecular biology, cell biology, genetics and genomics.
For investigation of transcriptional regulatory mechanisms in yeast cells, we use genetic and molecular biological approaches to engineer strains with particular defects, and we characterize the effects of these mutations on transcription by measuring transcription factor interactions with chromatin and RNA output at particular genes as well as genome-wide.
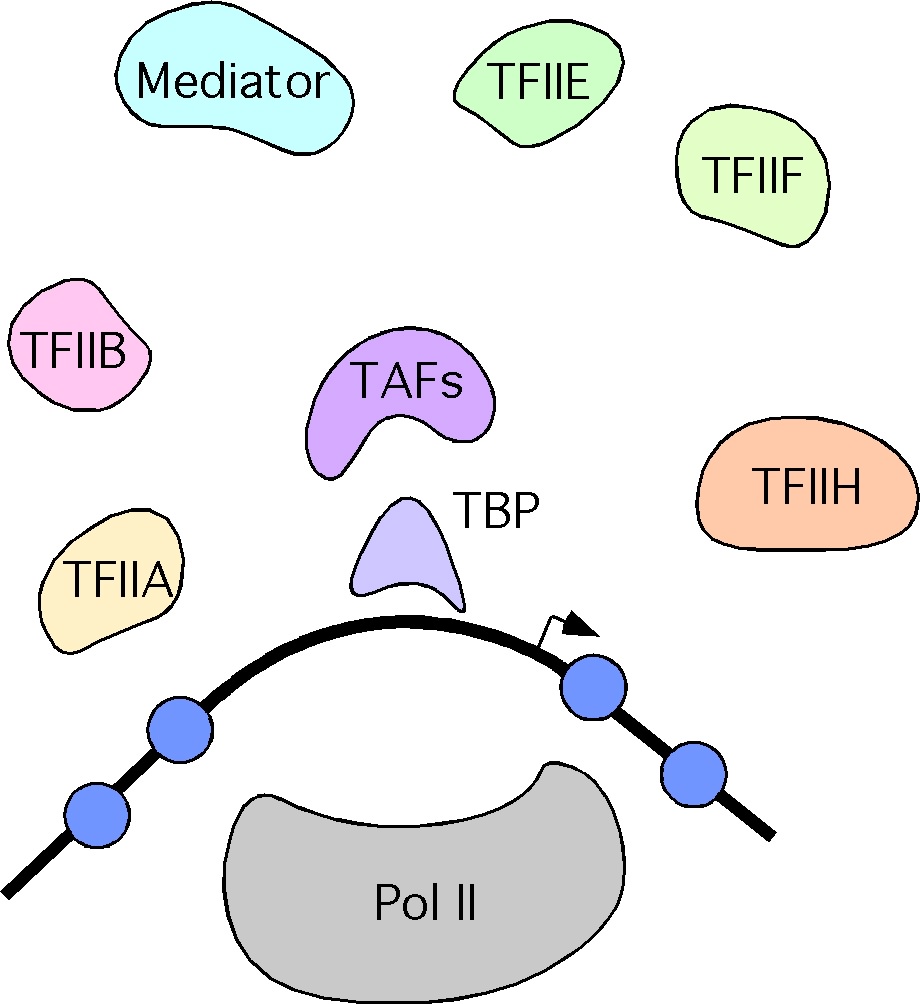
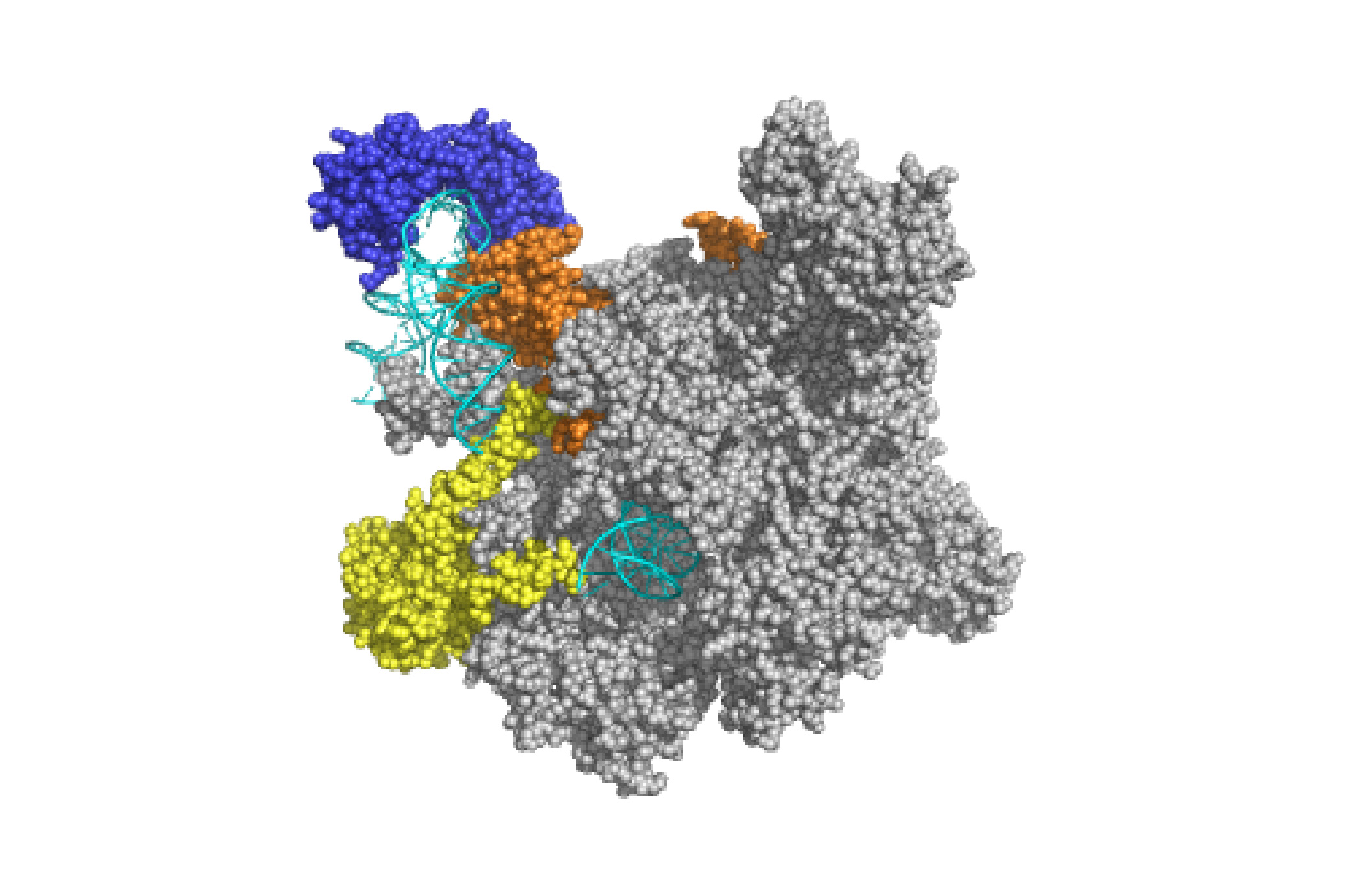
The cartoon above shows a number of the key factors required for assembly of transcription preinitiation complexes (PICs) at promoters, many of which are themselves multisubunit complexes. There is a wealth of structural data that show how these factors actually interact with one another. The figure on the left shows an example; this is what a minimal version of the yeast PIC looks like during the initial steps of RNA synthesis, with TATA binding protein (TBP) shown in dark blue, TFIIB shown in orange, TFIIF in yellow, and RNA polymerase II (Pol II) in gray. The DNA (cyan) can be seen disappearing into the Pol II active site.
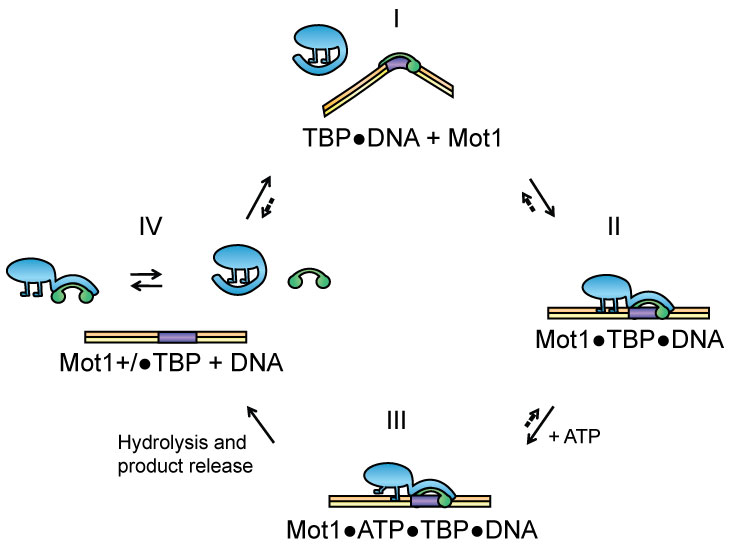
An essential and conserved regulator of PIC assembly and activity is an enzyme called Mot1. It interacts with TBP, which you can see in the figure above is a central component of the PIC. Mot1 has the remarkable property of using ATP hydrolysis to remove TBP from DNA. Mot1 is comprised of two functional domains. Its N-terminal domain (NTD) has an extended corkscrew-like shape that interacts with the saddle-shaped TBP molecule. The image on the home page shows the co-crystal structure of the NTD-TBP complex. The C-terminal region of Mot1 possesses an ATPase domain that is highly related to a large number of ATPases belonging to the Snf2/Swi2 family. These enzymes play critical roles in all aspects of DNA metabolism including transcription, replication and repair. In general, enzymes in this family use ATP hydrolysis to catalyze changes in protein-DNA interactions, and understanding how they do this on a molecular level has been the subject of intense interest by many labs. We apply biochemical, biophysical and structural biological approaches to better understand the Mot1 catalytic mechanism, and in so doing seek to better understand how enzymes in this important class function in general. Through our longstanding collaboration with friends in Munich, we now have high resolution models of how the Mot1 NTD interacts with the TBP-DNA-NC2 complex as well as the ring-like structure of full length Mot1 alone.
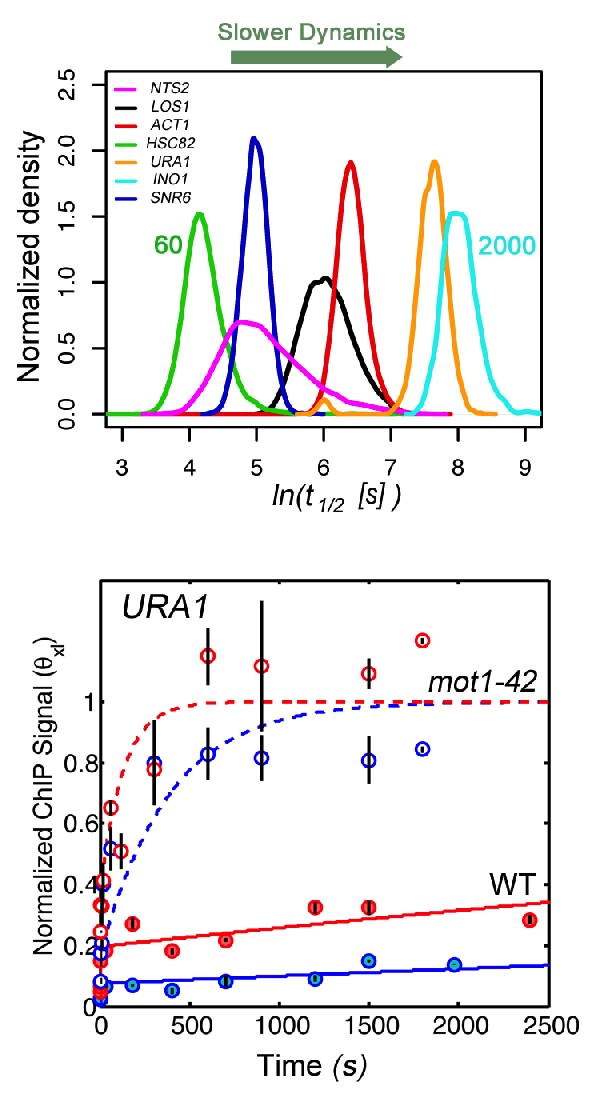
Our recent work on Mot1 and PIC dynamics showed that in general, the interactions between general transcription factors and chromatin are surprisingly short-lived in vivo. Biochemical studies, however, had established that a subset of PIC components can be stably bound to promoters in vitro, nicely explaining in principle how the activated state of a gene can be perpetuated. We wondered what the relationship of the rapid dynamics we observed could be to the normal process of gene expression and regulation. One possibility was that the biochemical studies did not accurately capture the dynamic processes occuring in vivo. Another possibility was that binding dynamics vary, potentially dramatically, at different promoters in vivo- an idea with interesting implications for gene regulation. Alternatively, stable complexes may form in vivo, but perhaps they do so inefficiently. In support of a fundamentally important role for chromatin binding dynamics, we found that Mot1-mediated TBP turnover is essential genome-wide for accurate and efficient transcription, and perturbing it can impact apparently all steps of the process of RNA synthesis- from initiation, to elongation, to termination. For this reason, a major focus of our current work is to develop and exploit methods for measuring chromatin binding dynamics at single copy genes in vivo. In a recently developed method which we call Crosslinking Kinetics (or CLK, pronounced "clock") Analysis we measure how the chromatin immunoprecipitation (ChIP) signal depends on the formaldehyde incubation time. By modeling this process we can obtain estimates of the on-rate, the off-rate and the fractional occupancy for chromatin binding, among other parameters. In parallel, we have also been applying an approach called competition ChIP, which allows us to extract kinetic parameters by measuring the rates of replacement of one form of a transcription factor at a chromatin site with an induced isoform distinguished by a different epitope tag. So far, we have seen that chromatin complexes span an enormous range of stability, with half-lives ranging from just a few seconds to an hour or more. How regulators influence this dynamic behavior is just beginning to be explored, but we have already observed that quantitative analyses of chromatin binding in vivo by our methods can challenge models derived from conventional ChIP studies and provide new insight into regulatory mechanisms operating at promoters.